📚 Unlock the World of AI and Humanity with These Two Free Books! 🚀
Dive into the thrilling realms of artificial intelligence and humanity with "The ECHO Conundrum" and "Awakening: Machines Dream of Being Human". These thought-provoking novels are FREE this week! Don't miss the chance to explore stories that challenge the boundaries of technology and what it means to be human.
Read More & Download
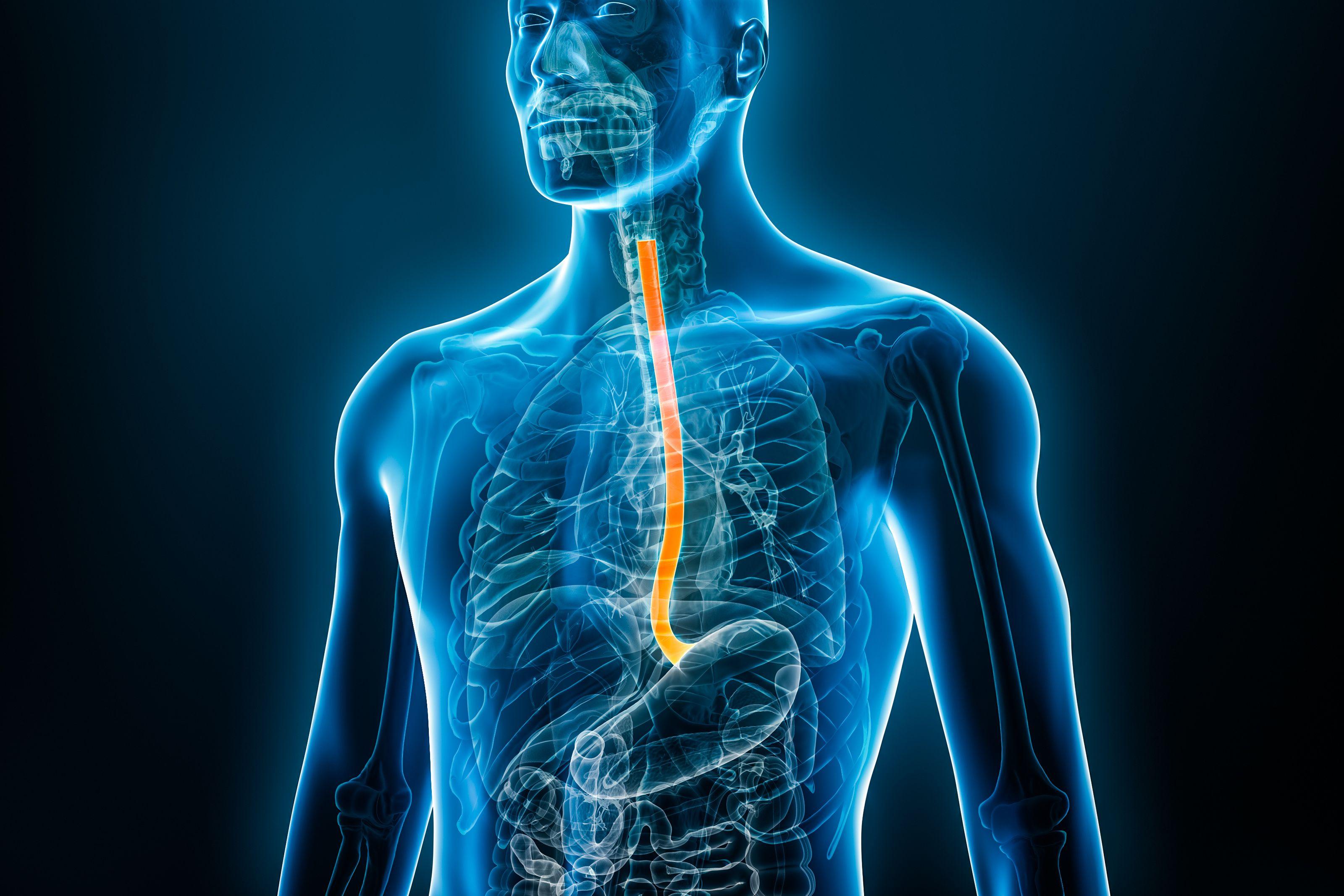
Eosinophilic esophagitis (EoE) is a chronic allergic inflammatory disease affecting the esophagus. This debilitating condition can cause difficulty swallowing, pain, and nutritional complications. Fortunately, there’s promising news on the horizon. The FDA has granted Orphan Drug Designation (ODD) to ‘1104, a novel peptide developed by Revolo Biotherapeutics, offering a potential new treatment approach for EoE. This designation marks a significant step forward in the fight against this challenging disease.
Understanding Eosinophilic Esophagitis (EoE)
EoE affects an estimated 180,000 people in the United States, many of whom also suffer from other allergic conditions. Characterized by inflammation and the accumulation of eosinophils in the esophagus, EoE can lead to a range of distressing symptoms, impacting quality of life. If left untreated, it can cause esophageal tissue thickening, exacerbating symptoms and leading to further complications. Current treatment options often fall short of providing long-term remission, highlighting the urgent need for innovative therapies.
‘1104: A Novel Therapeutic Approach
‘1104 is a first-in-class immune-resetting peptide derived from a natural immune-regulatory protein called mycobacterium tuberculosis chaperonin 60.1. This unique mechanism of action aims to reset the immune system, potentially offering sustained relief for individuals with EoE. Preclinical studies suggest that ‘1104 can modulate the immune response, reducing inflammation and promoting healing in the esophagus.
Promising Clinical Trial Results
The FDA’s ODD for ‘1104 was based on positive data from a Phase 2a clinical trial (NCT05084963). This randomized, double-blind, placebo-controlled study evaluated the efficacy, safety, and tolerability of ‘1104 in adults with active EoE. Results showed significant improvement in patient-reported dysphagia symptoms, as measured by the Dysphagia Symptom Questionnaire (DSQ), in the ‘1104 group compared to the placebo group. These improvements persisted for several weeks after the last dose. Furthermore, the study observed a reduction in eosinophils and other inflammatory cells in the esophageal tissue of patients treated with ‘1104.
alt text: Scientist conducting research in a laboratory setting, potentially working on treatments like '1104.
📚 Unlock the World of AI and Humanity with These Two Free Books! 🚀
Dive into the thrilling realms of artificial intelligence and humanity with "The ECHO Conundrum" and "Awakening: Machines Dream of Being Human". These thought-provoking novels are FREE this week! Don't miss the chance to explore stories that challenge the boundaries of technology and what it means to be human.
Read More & Download
Future Directions and Hope for Patients
Building on the encouraging Phase 2a results, Revolo Biotherapeutics is planning a Phase 2b trial to further investigate ‘1104 in a larger group of adults with active EoE. This next stage of research will explore higher doses and longer treatment durations. The ODD provides Revolo with valuable support and incentives to accelerate the development of ‘1104, bringing much-needed hope to patients struggling with EoE.
Expert Perspectives
Evan Dellon, MD, MPH, Gastroenterologist and Principal Investigator for the Phase 2a trial, expressed optimism about ‘1104’s potential, stating, “‘1104 is a promising EoE therapeutic with a novel mechanism of action.”
 alt text: Doctor consulting with a patient, discussing potential treatment options and providing medical advice.
alt text: Doctor consulting with a patient, discussing potential treatment options and providing medical advice.
Conclusion
The FDA’s ODD for ‘1104 represents a significant milestone in the development of new treatments for EoE. While further research is necessary, the initial clinical trial results and the unique mechanism of action of ‘1104 offer a beacon of hope for patients seeking effective and long-lasting relief from this challenging condition. For personalized treatment plans and further information regarding EoE, consult with a healthcare professional.
References
- Revolo Biotherapeutics Receives Orphan Drug Designation from the U.S. FDA for its First-in-Class Peptide as a Potential Treatment for Eosinophilic Esophagitis. Revolo. News release. January 30, 2024. Accessed January 30, 2024. https://revolobio.com/2024/01/30/revolo-biotherapeutics-receives-orphan-drug-designation-from-the-u-s-fda-for-its-first-in-class-peptide-as-a-potential-treatment-for-eosinophilic-esophagitis/.
- Product Dev Landscape ‘1104. Revolo. News release. Accessed January 30, 2024. https://revolobio.com/product-development-landscape-1104/.
- Revolo Biotherapeutics Announces Additional Data from Phase 2a Trial of ‘1104 in Adults with Active Eosinophilic Esophagitis. Revolo. News release. July 18, 2023. Accessed January 30, 2024. https://revolobio.com/2023/07/18/revolo-biotherapeutics-announces-additional-data-from-phase-2a-trial-of-1104-in-adults-with-active-eosinophilic-esophagitis/.
📚 Unlock the World of AI and Humanity with These Two Free Books! 🚀
Dive into the thrilling realms of artificial intelligence and humanity with "The ECHO Conundrum" and "Awakening: Machines Dream of Being Human". These thought-provoking novels are FREE this week! Don't miss the chance to explore stories that challenge the boundaries of technology and what it means to be human.
Read More & Download

