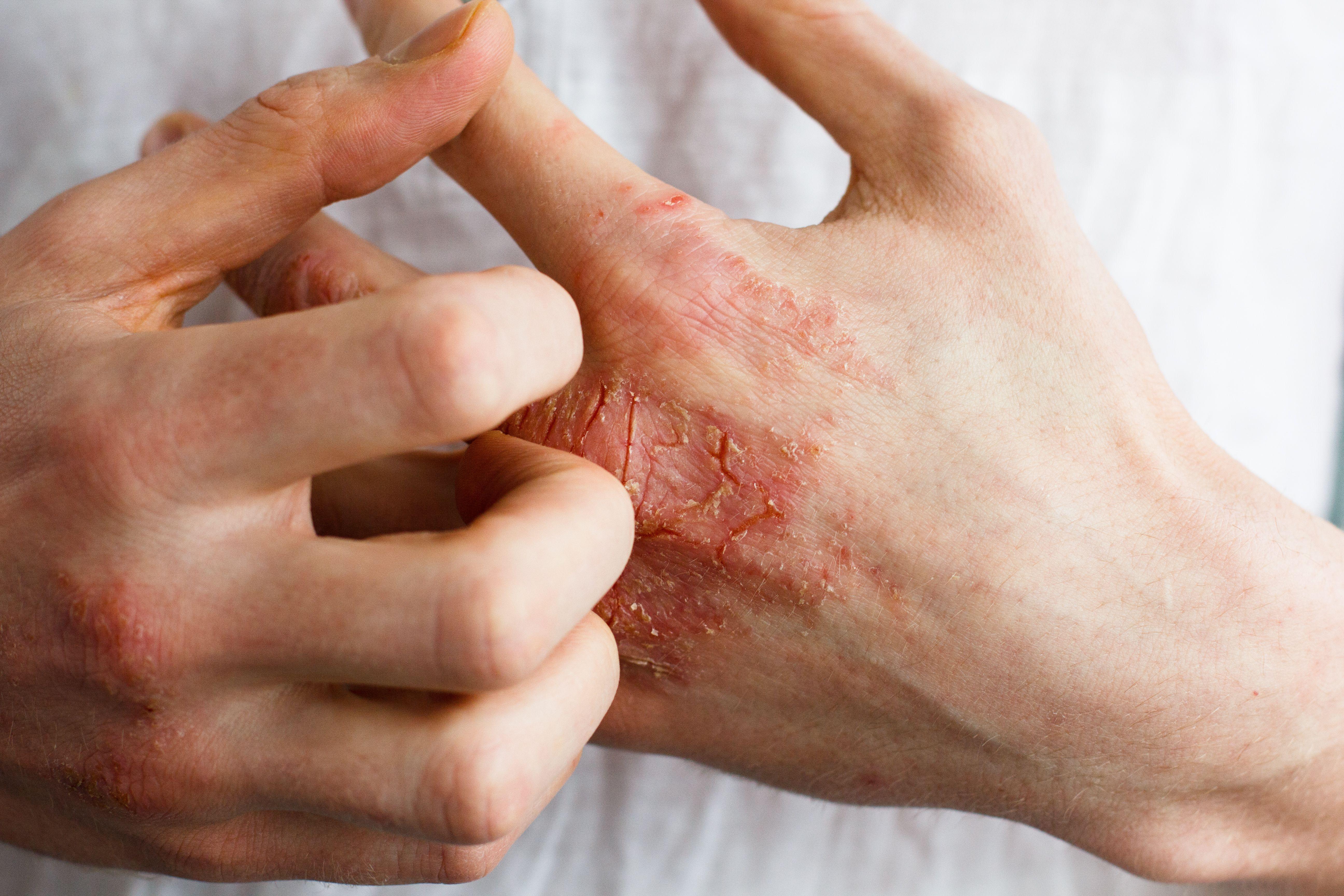
Chronic hand eczema (CHE) is a painful, itchy inflammatory skin condition affecting the hands and wrists, significantly impacting quality of life and occupational abilities. This condition, often persisting for over three months with frequent relapses, causes symptoms like itching, pain, redness, scaling, thickening of the skin, blisters, swelling, and cracks. A recent publication in The Lancet features groundbreaking research on a novel topical pan-Janus kinase (JAK) inhibitor, delgocitinib cream, offering potential relief for those suffering from this debilitating condition.
Image credit: Ольга Тернавская | stock.adobe.com
This marks the first time a topical pan-JAK inhibitor for CHE has been featured in the prestigious journal, signifying a significant step forward in understanding and treating this often-overlooked condition.
 A scientist working in a laboratory, symbolizing the research and development behind new eczema treatments.
A scientist working in a laboratory, symbolizing the research and development behind new eczema treatments.
Delgocitinib Cream: A New Approach to Treating CHE
The DELTA 1 and DELTA 2 trials, randomized, double-blinded, and vehicle-controlled phase 3 studies, investigated the efficacy and safety of twice-daily applications of delgocitinib cream (20 mg/g) in individuals with moderate to severe CHE. Delgocitinib targets the JAK-STAT signaling pathway, which plays a crucial role in the development of CHE.
Study Design and Participants
A total of 487 participants were enrolled in DELTA 1 and 473 in DELTA 2. In each trial, participants were randomly assigned to receive either delgocitinib cream or a vehicle control cream. The primary endpoint was treatment success as measured by the Investigator’s Global Assessment (IGA-CHE) score (0 for clear or 1 for almost clear) at week 16. Secondary endpoints included reduction in itch and pain scores.
Promising Results for Delgocitinib
The results, published in The Lancet, showed a significantly higher proportion of participants achieving IGA-CHE treatment success with delgocitinib cream compared to the vehicle control at week 16 in both trials. This suggests that delgocitinib cream could be an effective treatment option for individuals with moderate to severe CHE.
Safety Profile of Delgocitinib Cream
The safety profile of delgocitinib cream was also assessed. The proportion of reported adverse events was similar between the delgocitinib and vehicle groups. The most common adverse events, occurring in at least 2% of participants in both groups, included COVID-19 and nasopharyngitis.
Implications for Future CHE Management
The publication of these findings in The Lancet is a significant milestone, raising awareness of CHE and underscoring the importance of continued research in this area. It signifies a promising step towards developing effective and safe treatment options for individuals living with this chronic condition.
Conclusion: A New Hope for CHE Sufferers
Delgocitinib cream demonstrates potential as a novel treatment for moderate to severe CHE. While further research is warranted, these positive phase 3 trial results offer hope for improved management of this challenging condition. For personalized treatment plans and to discuss if delgocitinib is right for you, consult with a dermatologist or healthcare professional.
References
LEO Pharma Announces Publication of Chronic Hand Eczema Phase 3 Data in The Lancet. Leo Pharma. News release. July 19, 2024. Accessed July 25, 2024.
Bissonnette R, et al. Efficacy and safety of delgocitinib cream in adults with moderate to severe chronic hand eczema (DELTA 1 and DELTA 2): results from multicentre, randomised, controlled, double-blind, phase 3 trials. The Lancet. July 18, 2024. Accessed July 25, 2024. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(24)01027-4/abstract.

