📚 Unlock the World of AI and Humanity with These Two Free Books! 🚀
Dive into the thrilling realms of artificial intelligence and humanity with "The ECHO Conundrum" and "Awakening: Machines Dream of Being Human". These thought-provoking novels are FREE this week! Don't miss the chance to explore stories that challenge the boundaries of technology and what it means to be human.
Read More & Download
Acute lymphoblastic leukemia (ALL) is a challenging cancer, especially when it relapses or becomes resistant to treatment (R/R). This article examines the FDA-approved drug inotuzumab ozogamicin, a significant advancement in treating pediatric R/R B-cell ALL, and explores the evolving therapeutic landscape for this disease.
Understanding Acute Lymphoblastic Leukemia (ALL)
ALL is a heterogeneous disease marked by the uncontrolled growth of immature lymphoid cells in the bone marrow, blood, and other organs. While rare, it predominantly affects children under five. Diagnosis involves blood tests, bone marrow biopsies, and immunophenotyping to identify specific cell markers. Genetic abnormalities, such as chromosomal translocations, play a crucial role in prognosis and treatment strategies. For example, the t(12;21) translocation is associated with a favorable prognosis in children, while the Philadelphia chromosome (Ph+) signifies a poorer outcome.
 Illustration depicting the process of chromosomal translocation in acute lymphocytic leukemia.
Illustration depicting the process of chromosomal translocation in acute lymphocytic leukemia.
Treatment Approaches for Pediatric ALL
Treatment for ALL is complex, typically involving intensive chemotherapy phases: induction, consolidation, and maintenance. Induction aims to achieve remission, using drugs like vincristine, corticosteroids, and asparaginase, often with anthracyclines. Consolidation intensifies treatment to eliminate residual leukemic cells, while maintenance therapy aims to prevent relapse. Central nervous system (CNS) prophylaxis is essential due to the risk of leukemic cells spreading to the brain and spinal cord. Targeted therapies, including monoclonal antibodies and CAR T-cell therapies, have revolutionized treatment, particularly for R/R ALL.
Inotuzumab Ozogamicin: A Targeted Therapy for R/R ALL
Inotuzumab ozogamicin, an antibody-drug conjugate (ADC), targets the CD22 protein found on B-cells. The antibody delivers a potent cytotoxic agent directly to the leukemia cells, minimizing damage to healthy tissues. The FDA approved inotuzumab ozogamicin for R/R B-cell ALL in adults in 2017 and extended the approval to pediatric patients aged one and older in 2024. Clinical trials, like the INO-VATE ALL and ITCC-059 studies, demonstrated promising complete remission (CR) rates and minimal residual disease (MRD) negativity in pediatric patients.
Clinical Trial Results and Efficacy
The INO-VATE ALL trial showed a 42% CR rate with a median duration of 8.2 months. The ITCC-059 study, a phase 2 trial, established the recommended dose at 1.8 mg/m2 per cycle, achieving an 85% CR rate in patients with multiple relapses. These trials, along with other studies like COG AALL1621, have confirmed the efficacy and safety of inotuzumab ozogamicin in pediatric R/R B-cell ALL. While hepatotoxicity, including sinusoidal obstruction syndrome (SOS), is a significant concern, particularly after hematopoietic stem cell transplantation (HSCT), the benefits of this targeted therapy often outweigh the risks.
📚 Unlock the World of AI and Humanity with These Two Free Books! 🚀
Dive into the thrilling realms of artificial intelligence and humanity with "The ECHO Conundrum" and "Awakening: Machines Dream of Being Human". These thought-provoking novels are FREE this week! Don't miss the chance to explore stories that challenge the boundaries of technology and what it means to be human.
Read More & Download
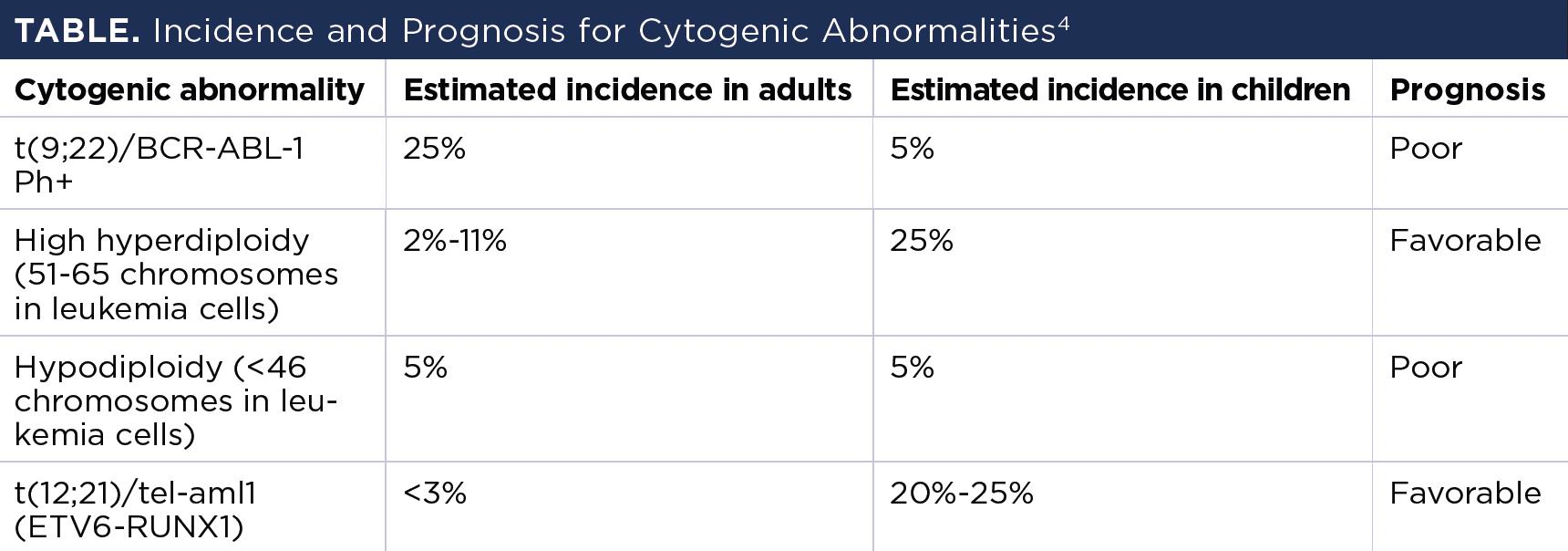 Table showing the incidence and prognosis for different cytogenic abnormalities in acute lymphoblastic leukemia.
Table showing the incidence and prognosis for different cytogenic abnormalities in acute lymphoblastic leukemia.
Other Treatment Options for R/R ALL
Other immunotherapies for R/R ALL include blinatumomab, a bispecific T-cell engager, and tisagenlecleucel, a CAR T-cell therapy. While these therapies have shown positive results, inotuzumab ozogamicin offers a valuable alternative, especially for patients who are not eligible for or have not responded to other treatments.
Ongoing Research and Future Directions
Ongoing research focuses on combining inotuzumab ozogamicin with chemotherapy regimens, such as the Pedi-cRIB trial, to further improve outcomes in pediatric R/R ALL. These trials aim to optimize treatment protocols and address challenges like drug resistance and antigen loss.
Conclusion
Inotuzumab ozogamicin represents a significant advance in the treatment of pediatric R/R B-cell ALL. This targeted therapy offers a new hope for patients who have exhausted other treatment options. Ongoing research and clinical trials are paving the way for further advancements in managing this complex disease. For personalized treatment plans, consult with a healthcare professional.
📚 Unlock the World of AI and Humanity with These Two Free Books! 🚀
Dive into the thrilling realms of artificial intelligence and humanity with "The ECHO Conundrum" and "Awakening: Machines Dream of Being Human". These thought-provoking novels are
FREE this week! Don't miss the chance to explore stories that challenge the boundaries of technology and what it means to be human. Read More & Download

