📚 Unlock the World of AI and Humanity with These Two Free Books! 🚀
Dive into the thrilling realms of artificial intelligence and humanity with "The ECHO Conundrum" and "Awakening: Machines Dream of Being Human". These thought-provoking novels are FREE this week! Don't miss the chance to explore stories that challenge the boundaries of technology and what it means to be human.
Read More & Download
The COVID-19 pandemic underscored the critical need for effective vaccines to protect vulnerable populations, including pregnant and lactating women. This in-depth analysis explores a cohort study examining the immunogenicity and reactogenicity of mRNA COVID-19 vaccines in these groups compared to non-pregnant women and those with natural COVID-19 infection during pregnancy.
This study provides crucial data on antibody responses to vaccination, compares vaccine-induced immunity with natural infection, and assesses antibody transfer to newborns. The findings have significant implications for vaccination strategies and public health recommendations.
Materials and Methods
The prospective cohort study enrolled 131 women: 84 pregnant, 31 lactating, and 16 non-pregnant controls. Antibody levels in serum and breastmilk were measured at various time points: baseline, second vaccine dose, 2-6 weeks post-second dose, and delivery. Umbilical cord blood was also analyzed. A comparison group included 37 pregnant women with natural SARS-CoV-2 infection.
Results
Robust Antibody Response to Vaccination
mRNA vaccination elicited a significant increase in all antibody isotypes (IgG, IgM, IgA) against SARS-CoV-2 spike and receptor-binding domain (RBD) proteins.
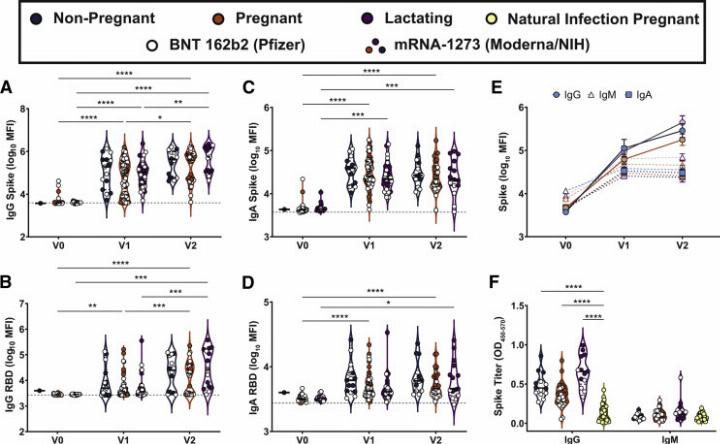 Maternal vaccination induces a robust SARS-CoV-2–specific antibody response
Maternal vaccination induces a robust SARS-CoV-2–specific antibody response
Antibody titers in pregnant and lactating women were comparable to those in non-pregnant women and substantially higher than in naturally infected pregnant women. The second vaccine dose (booster) further enhanced IgG levels but not IgM or IgA.
Antibody Transfer to Neonates
Crucially, vaccine-induced antibodies were detected in all umbilical cord blood and breastmilk samples.
📚 Unlock the World of AI and Humanity with These Two Free Books! 🚀
Dive into the thrilling realms of artificial intelligence and humanity with "The ECHO Conundrum" and "Awakening: Machines Dream of Being Human". These thought-provoking novels are FREE this week! Don't miss the chance to explore stories that challenge the boundaries of technology and what it means to be human.
Read More & Download
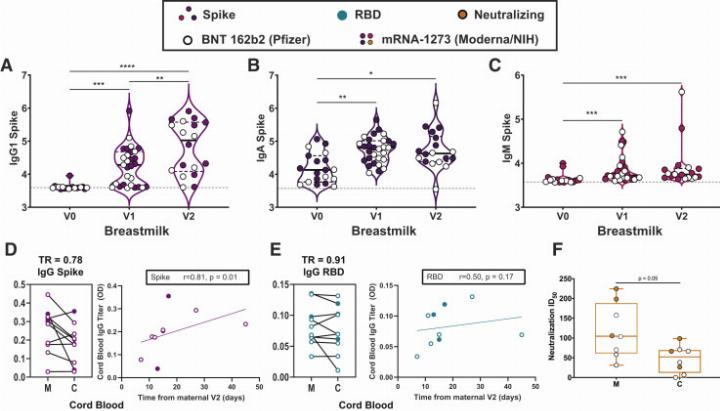 Placental and breastmilk transfer of vaccine-induced SARS-CoV-2 antibodies
Placental and breastmilk transfer of vaccine-induced SARS-CoV-2 antibodies
While neutralizing antibody titers were lower in cord blood than maternal serum, the difference was not statistically significant. IgG transfer to breastmilk increased after the booster dose, suggesting enhanced neonatal protection.
Discussion
This study demonstrates that mRNA COVID-19 vaccines generate a robust humoral immune response in pregnant and lactating women, comparable to non-pregnant individuals and superior to natural infection. Furthermore, antibodies are efficiently transferred to newborns via both placenta and breastmilk, potentially providing crucial early protection.
The findings support the recommendation of COVID-19 vaccination for pregnant and lactating women, given the known risks of severe COVID-19 in pregnancy and the potential for maternal antibodies to protect newborns.
Conclusion
This study provides compelling evidence of the efficacy and safety of mRNA COVID-19 vaccines in pregnant and lactating women. The robust antibody responses and demonstrated transfer of immunity to newborns underscore the importance of vaccination in this population. Future research should focus on long-term immunity and the optimal timing of vaccination during pregnancy.
📚 Unlock the World of AI and Humanity with These Two Free Books! 🚀
Dive into the thrilling realms of artificial intelligence and humanity with "The ECHO Conundrum" and "Awakening: Machines Dream of Being Human". These thought-provoking novels are FREE this week! Don't miss the chance to explore stories that challenge the boundaries of technology and what it means to be human.
Read More & Download

